Prof. Nerurkar Awarded Major $1.9M Embryo Grant
The 4-year grant from the National Institutes of Health (NIH) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) will support Prof. Nerurkar's research into the biophysical and biochemical intricacies of how embryos precisely sculpt healthy organs, such as the small intestine, during development.
Professor Nandan Nerurkar has earned a major four-year grant totaling $1.9 million from the National Institutes of Health (NIH) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The grant, entitled "Molecular control of mechanical forces driving buckling morphogenesis of the small intestine" will support his research into the biophysical and biochemical intricacies of how embryos precisely sculpt healthy organs, such as the small intestine, during development.
A member of the department of Biomedical Engineering, Nerurkar investigates how tissues and organs form in developing embryos via interwoven genetic, molecular, and biophysical cues. Utilizing live in vivo imaging of early-stages in a classical embryology model system, the chicken embryo, his Morphogenesis and Developmental Biomechanics Lab explores how developmental signals specify the forces that influence tissue growth and stem cell differentiation, including in birth defects when such processes go awry. Ultimately, he seeks to uncover design principles of embryonic tissue formation for applications in regenerative medicine and tissue engineering.
The grant will support Nerurkar’s work in building highly quantitative perspectives on the many factors affecting embryonic development, particularly in understanding how cell behaviors in the developing small intestine are regulated by molecular cues, and how the resulting mechanical events that shape this organ feed back to control these cell behaviors, ensuring stereotyped morphogenesis of this and other organs in the developing embryo. These studies will provide important insight into the basis of devastating birth defects of the small intestine, and will reveal mechanisms for controlling tissue self-organization that can be repurposed for regenerative medicine and tissue engineering applications.
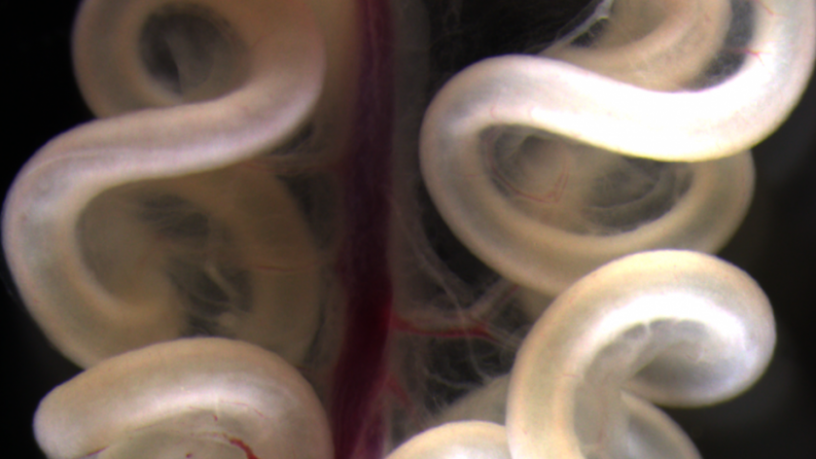
The small intestine of a 16 day chick embryo. The precise loops are essential for packing the lengthy intestinal tube within the body cavity, and abnormal looping in humans results in devastating birth defects such as volvulus and gastroschisis.